BIA-SCC: Rare reports of breast implant-associated squamous cell carcinoma
In September 2022, the Food and Drug Administration (FDA) posted a Safety Communication on their website informing healthcare practitioners and consumers about a number of reports received of squamous cell carcinoma and various lymphomas (not including BIA-ALCL) detected in the capsule surrounding breast implants.
While this announcement took us all by surprise, it’s important to put these findings of breast implant-associated squamous cell carcinoma (BIA-SCC) into perspective. I personally have not seen any cases of breast implant-associated tumors, including BIA-SCC or BIA-ALCL since starting my private practice in 2006, despite performing thousands of breast implant surgeries.

I only offer smooth surfaced implants in my practice. Our patients are not at risk of BIA-ALCL, which has been reported to be associated with textured implants.
Since breast implants were brought to market over 50 years ago, 16 cases of squamous cell carcinoma associated in some way with breast implants have been detected and reported to the MAUDE database, which was created over 30 years ago in 1991 for the purposes of reporting adverse events related to medical devices. MAUDE stands for the “Manufacturer and User Device Experience” database, which houses information about adverse medical device incidents (malfunctions, injuries, deaths) that are submitted to the FDA . These reports are submitted by facilities, manufacturers, and users such as physicians or self-reports by patients or consumers. It is mandatory for manufacturers such as breast implant companies to report incidents when they become aware of them.
BIA-SCC is known to occur with both type of surfaces (textured and smooth) and filler (silicone and saline), whereby BIA-ALCL has only been associated with textured implants. Based on the literature there appears to be a bias to implants in place for many years (several decades). The literature associates chronic inflammation associated with device associated infection as the driver of the process like Marjolijn’s Ulcer where a squamous cell carcinoma of the skin can occur decades after a past burn injury.
Current BIA-SCC cases reported include patients diagnosed after years of having breast implants placed and have all presented with abnormal signs and symptoms including swelling, pain, lumps, or skin changes, as in the photograph from a reported case of BIA-SCC below. Awareness by breast implant patients and physicians is important, as well as emphasis on the need for long-term patient follow-up with their Plastic Surgeon.
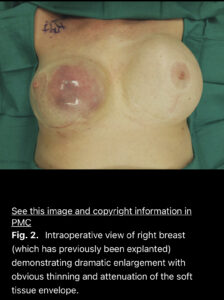
Example of a breast implant-associated squamous cell carcinoma arising in the capsule of an implant placed 35 years previously. Click the photo for a link to the full scientific article.
Our professional societies including The Aesthetic Society (formerly ASAPS) and American Society of Plastic Surgeons (ASPS) have issued statements regarding BIA-SCC that I am sharing with you. It is a LOT of information – feel free to scan it or read it in detail. We will update this blog post as more information/research becomes available in the future.
From The Aesthetic Society:
September 13, 2022
The Aesthetic Society developed the following points about BIA-SCC:
- The FDA advisory is not new information nor is it unique to breast implants where multiple rare malignancies have been reported around all types of implants including orthopedic implants, dental implants, pacemakers, and breast implants. The incidence of these malignancies including BIA-SCC is rare.
- Awareness for physicians and patients is key. ANY abnormalities and deviation from the normal course for implant patients should be evaluated by a Board-Certified Plastic Surgeon.
- Strongly continue to recommend that breast implant patients have yearly follow-up and report any changes to their surgeon.
- Asymptomatic implant patients should have their first breast ultrasound or MRI about 5-6 years postoperatively, then every 2-3 years thereafter.
- BIA-SCC official treatment recommendations will need to be based on emerging data, and we will continue to update you.
- For this initial communication we recommend the following guidelines:
- The presentation of BIA-SCC has included breast/axillary mass, unilateral swelling, pain, erythema.
- Surgeons should be aware of both BIA-ALCL, BIA-SCC or other possible malignancies when evaluating implant patients for changes in their breasts and should not operate on anyone with a seroma or mass without an appropriate pre-operative work up.
- For late seroma the diagnostic tool of choice is an ultrasound. Fluid should be aspirated and sent requesting the pathologist to rule out malignancy.
- If you have a patient that has a late seroma, preoperatively fluid should be sent for cytospin or cellblock and CD 30 testing. Based on this evaluation the pathologist will be able to decide if any further testing is warranted. The majority of these cases will have a negative evaluation as most late seromas are non-malignant. Despite, this, these are typically treated with surgical therapy. The capsule should be inspected and any suspicious areas submitted for histologic, and when appropriate, immunohistochemical evaluation, e.g. Cd5/6, p65 for suspicion of squamous cell carcinoma.
The Aesthetic Society Leadership shared a roundtable discussion about the FDA alert for BIA-SCC here:
The following is information from the ASPS:
ASPS statement on Breast Implant Associated-Squamous Cell Carcinoma (BIA-SCC)
On Sept. 8, 2022, the Food and Drug Administration (FDA) released a new safety communication about squamous cell carcinoma (SCC) and various lymphomas in the capsule around breast implants. This document is now available to healthcare providers, patients and caregivers on the FDA’s Medical Device Safety webpage.
The American Society of Plastic Surgeons (ASPS)/The Plastic Surgery Foundation (PSF) has been in communication with the Food and Drug Administration (FDA) regarding this emerging issue. Data on this topic are limited and evolving; however, the Society would like to provide ASPS members with additional information specifically about Breast Implant-Associated Squamous Cell Carcinoma (BIA-SCC) to support increased clinical awareness and enhanced clinical decision-making as more evidence becomes available.
BIA-SCC is a very rare but potentially aggressive epithelial-based tumor that appears to be associated with breast implants and emanates from the breast implant capsule. At this time, ASPS/PSF is aware of so few reported cases of BIA-SCC that it is not possible to determine what factors increase patient risk.
ASPS/PSF is committed to driving patient safety and informed decision making through research and the ongoing and persistent surveillance of breast implants. Information for your patients may be found on the ASPS webpage (Breast Implant Safety: What Patients Need to Know).
Overview
The following overview is presented to help Plastic Surgeons recognize Breast Implant-Associated Squamous Cell Carcinoma (BIA-SCC) as a distinct disease entity and long-term complication of breast implants. In light of broad specialty-wide awareness concerning Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL), information regarding BIA-SCC is presented in a comparative format below. A reference list of all available published case reports is included at the end of this statement, and The PSF is finalizing a manuscript summarizing the current state of knowledge for Plastic and Reconstructive Surgery.
| BIA-SCC | BIA-ALCL | |
| What is it? | Breast implant-associated squamous cell carcinoma (BIA-SCC) is a very rare but potentially aggressive, epithelial-based tumor that appears to emanate from the breast implant capsule. Pathology shows sheets of squamous cells lining the capsule in nests and bundles. BIA-SCC can exhibit highly invasive properties including spread to lymph nodes, local tissues and distant sites, such as muscle and bone. BIA-SCC is not a cancer of the breast tissue itself. | Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon and treatable type of T-cell lymphoma that can develop around breast implants. BIA-ALCL can exhibit highly invasive properties, including spread to lymph nodes, local tissues and distant sites. BIA-ALCL is not a cancer of the breast tissue itself. |
| Number of Known Cases | To the best of our knowledge, there are 16 cases reported in the literature; 2 additional cases have been reported to The PSF from individual surgeons and are under review. | ASPS recognizes approximately 400 both suspected and confirmed cases in the United States and a total of 1,227 worldwide as of August 2022. |
| Lifetime Risk | Unknown | Current lifetime risk of BIA-ALCL varies widely (e.g., estimates of 1:2,207-1:86,029 based upon variable risk with different manufacturer types of textured implants. More recently, cumulative risk over 20 years in breast reconstruction patients implanted with Biocell devices was estimated at 1:100 (Cordeiro et al, 2020). |
| Age at presentation | 55.8 years (range 40-81) | 55.3 years (range 28-84) |
| Average length since initial implantation | 22.74 years (range 11-40 years) | 10.32 years (range 0.08-41 years) |
| Implant Surface | In case reports, BIA-SCC has been reported in patients who have had smooth and/or textured implants. | No cases of BIA-ALCL have been confirmed in patients who have only had smooth implants in case series, case reports or registries. However, it is not possible to exclude the appearance of BIA-ALCL in association with smooth implants at this time. The FDA states that all confirmed cases worldwide either have a history of a textured device or an incomplete clinical history available for review. |
| Implant Type | BIA-SCC has been associated with both silicone and saline implants in aesthetic as well as reconstructive patients. | BIA-ALCL has been associated with both silicone and saline implants in aesthetic as well as reconstructive patients. |
| Presentation | ||
| • Delayed seroma | Yes | Yes |
| • Unilateral swelling | Yes | Yes |
| • Pain, erythema | Yes | Yes |
| • Capsular contracture | Often | Sometimes |
| Extracapsular spread at presentation | 80% at presentation | 28% at presentation |
| Typical Pathology | Squamous cells in sheets with varying degrees of atypia and metaplasia and at least one focus of SCC. | Lymphoma with mass confined to single area on capsule. |
| Diagnostic Assessment | CK 5/6+; p63+; Flow cytometry + for squamous cells and keratin | CD30+; ALK-; Flow cytometry + for T-cells |
| Imaging | Ultrasound to evaluate for peri-prosthetic fluid +/- aspiration; MRI with and without contrast to evaluate capsule to rule out mass; PET-CT for extent of disease, if present. | Ultrasound to evaluate for peri-prosthetic fluid +/- aspiration; PET‐CT is performed following a positive diagnosis. Mammograms are not helpful for evaluating lymphoma but are important for the evaluation of breast cancer. |
| Treatment | Official treatment recommendations will need to be based on emerging data. At present, it appears that explantation with complete (en bloc) capsulectomy will provide the best outcomes. Based on existing case reports, it appears that incomplete resection of BIA-SCC can result in early and/or aggressive recurrence. | In the majority of cases, explantation with complete (en bloc) capsulectomy is curative. Incomplete capsular resection has been associated with both recurrence and significantly lower survival. Rare patients will present with more advanced disease and may require radiotherapy and chemotherapy. Treatment approach should follow international guidelines established by the National Comprehensive Cancer Network (NCCN) for BIA-ALCL. Current treatment recommendation is for bilateral complete capsulectomy and implant removal, as a small number of women have had contralateral disease found incidentally. |
| Chemotherapy / Radiation Therapy | Patients treated within these cases did not appear to respond. | Responds to Brentuximab plus CT. |
| Mortality | 43.8% at six months. | 2.8% at one year. |
| Reporting | The FDA recommends that any suspected or confirmed cases of SCC, lymphomas, or any other cancers around the breast implant be reported to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database and the device manufacturer. To submit a case to the MAUDE database, which collects medical device reports (MDRs) of suspected device-associated deaths, serious injuries and malfunctions, visit www.accessdata.fda.gov. Pending IRB approval, The PSF will soon enable plastic surgeons to report confirmed or suspected breast implant-associated capsular pathologies, including BIA-SCC cases, to PROFILE (ThePSF.org/PROFILE) | The FDA recommends that any suspected or confirmed cases of BIA‐ALCL be reported to the PROFILE registry, the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database and the device manufacturer. To submit a case to the MAUDE database, which collects medical device reports (MDRs) of suspected device-associated deaths, serious injuries and malfunctions, visit www.accessdata.fda.gov. To report a case to PROFILE, go to ThePSF.org/PROFILE. |
| Patient Counseling and Informed Consent | BIA‐SCC should be discussed with any patient considering breast implants as part of the informed-consent process. | BIA‐ALCL should continue to be discussed with any patient considering breast implants as part of the informed-consent process. |
ASPS/PSF Recommendations
- Prior to implantation of any breast implant, Plastic Surgeons should provide patients with the manufacturer’s patient labeling, the FDA-required patient decision checklist and any other educational material to best discuss the benefits and risks of breast implants.
- Preoperative workup is essential. All patients presenting with a late seroma should have fine needle aspiration (FNA) and cytology testing. Specimens should be sent for immunohistochemistry including CD30, ALK, CK 5/6, p63 and flow cytometry to look for T-cells, squamous cells and keratin.
- All patients presenting with a late seroma should undergo a breast ultrasound and MRI with and without contrast. If disease is confirmed, a PET-CT should be considered prior to surgical intervention. A thorough preoperative work-up allows for potentially the most appropriately planned, single-stage surgery with the greatest chance of success for cure.
- Consider the possibility of BIA-ALCL, BIA-SCC and other lymphomas when treating a patient with late onset, peri-implant changes. If you have a patient with suspected BIA-ALCL or BIA-SCC, refer them to experts familiar with the diagnosis and treatment of BIA-ALCL and BIA-SCC.
- At surgery, collect fresh seroma fluid, representative portions of the capsule, and specific pathology requests to rule out both BIA-ALCL and BIA-SCC.
- Diagnostic evaluation should include cytological evaluation of seroma fluid or mass with Wright Giemsa stained smears and cell block immunohistochemistry/flow cytometry testing for cluster of differentiation (CD30) and Anaplastic Lymphoma Kinase (ALK) markers, as well as Cytokeratin 5/6 (CK 5/6) and p63.
- Flow cytometry should include instructions to look for T cells, squamous cells, and keratin.
- Diagnostic evaluation should include cytological evaluation of seroma fluid or mass with Wright Giemsa stained smears and cell block immunohistochemistry/flow cytometry testing for cluster of differentiation (CD30) and Anaplastic Lymphoma Kinase (ALK) markers, as well as Cytokeratin 5/6 (CK 5/6) and p63.
- All confirmed or suspected BIA-SCC data should be entered into the PROFILE Registry (Data entry mechanism forthcoming).
- Data for all patients with seroma should be entered into the National Breast Implant Registry (NBIR).
ASPS/PSF is committed to patient safety, advancing quality of care and practicing medicine based upon the best available scientific evidence. We will continue to monitor and review all new information as it becomes available to keep the plastic surgery community informed. If you have any further Questions, please do not hesitate to contact Amy Hughes at ahughes@plasticsurgery.org.
We are committed to patient education and lifelong patient safety in our San Francisco Plastic Surgery practice. If you have questions or concerns about your breast implants, please contact my office or call 415-923-3067 to speak to someone on my team directly and to schedule an in-person assessment.




